Nox T3s
Polygraph with sleep time estimation
Like its predecessor, the Nox T3s is an easy-to-use, portable sleep recorder that enables the home-based diagnosis of sleep-related breathing disorders. With Nox BodySleep, a new and accurate sleep indicator, along with a modernised version of the Noxturnal software which offers an intuitive results overview and visualisation of the sleep states, the Nox T3s ensures accurate diagnostics.




Discover Nox T3s
The Nox T3s is an intuitive Home Sleep Test device with easy-to-use features, an updated design and a responsive user interface that patients and healthcare professionals will find easy to use. Watch this video to learn more.
Nox BodySleep: The first sleep indicator
Nox BodySleep is a new function that adopts an Artificial Intelligence (AI) method which classifies each 30-second time frame as a state of REM sleep, NREM sleep or wakefulness. This approach was mainly implemented by extracting information from the Nox RIP belts in the Nox A1 PSG studies1.
Noxturnal: Patient data management software
Noxturnal has been modernised with a new interface to better adapt to each clinician’s specific requirements and allow even more effective management of patient data. This is achieved through:
- Nox BodySleep analysis improving AHI accuracy1.
- Updated PLM analysis updated for more precise diagnostics1.
- New recording results overview designed to provide workflow efficiency.
- New report parameters calculations giving physicians more in-depth data and report customisation flexibility.

Warranty and services
Looking for service and warranty information on ResMed Nox T3s? Find the answers to your questions in our resource centre.
Setting patients up with Nox T3s
Nox T3s FAQ
The Nox T3s retains all the advantages of the latest Nox T3 device, with the addition of the following enhanced features:
- The new Nox BodySleep, an accurate sleep indicator giving an estimation of the sleep states (REM sleep, NREM sleep and wakefulness).
- New, updated version of Noxturnal software offering advanced functionalities adapted to the new device features.
- Improved design for better cleaning and efficiency.
More intuitive with a modernised interface, the Noxturnal v6 offers a wide range of new features:
- Nox BodySleep analysis improving AHI accuracy.
- PLM analysis updated for more precise diagnostics.
- New recording results overview designed to provide workflow efficiency.
- New report parameters calculations giving the physician more data depth and report customisation flexibility.
Nox BodySleep uses an advanced method to measure the physiological impact of brain state changes on the body. The sleep indicator:
- Estimates the states of REM sleep, NREM sleep and wakefulness.
- Is based on extracting information from the Nox RIP belts in the Nox A1 PSG studies1.
- Provides a more efficient analysis of Sleep Disordered Breathing for accurate diagnostics.
To evaluate the reliability of the Nox BodySleep algorithm, Nox Medical used a method1 based on the calculation of two coefficients:
- F1-score measuring accuracy of a test. This coefficient reaches its best value to 1, considered as perfect precision. The state-of-art score is 0.804.
- The Cohen’s Kappa measuring inter-rater reliability between different raters. This coefficient is considered substantial when it’s in the range of 0.61 – 0.803. In this case, the raters are sleep types (Wake, NREM and REM).
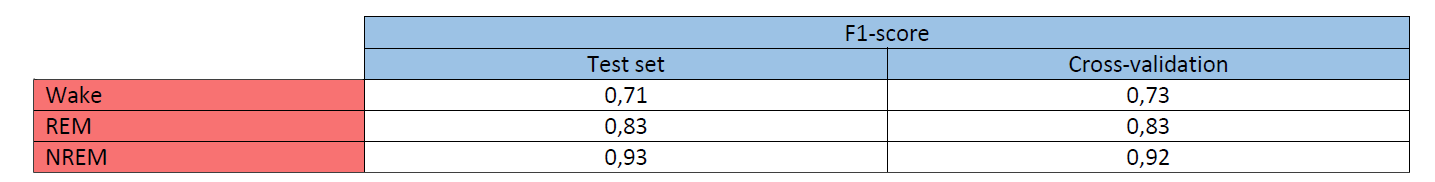
Their method was evaluated on the clinical PSG dataset, using a five-fold cross-validation and a hidden test set, given the following results
After calculation, the average F1-score = 0.88 and Cohen‘s Kappa = 0374 for the test set and 0.75 for cross validation. These coefficients are therefore considered as substantial, and thus the Nox BodySleep algorithm as reliable.
In addition, further tests2 were conducted to demonstrate the accuracy of the Nox BodySleep algorithm. This validation was performed in comparison with polysomnography recordings, in a sleep laboratory including patients with sleep disordered breathing and sleep related movements disorders.
The results were analysed by descriptive statistics methods (IBM SPSS Statistics 25.0), given an overall accuracy of 0.8 and a Cohen’s Kappa of 0.7. Considering these coefficients as substantial, the authors of the study conclude that the algorithm shows good diagnostic accuracy for the estimation of sleep states and significant sleep parameters.
No, Nox BodySleep is not considered as a method for directly measuring brain states associated with wake and sleep but is an advanced feature estimating the different states of sleep (REM, NREM sleep and wakefulness). It may not replace PSG hypnogram-based clinical decisions.
Nox T3s technical specifications
Looking for service and warranty information on ResMed Nox T3s? Find the answers to your questions in our resource centre.
- Available Signals
- Thorax and abdomen RIP
- Nasal pressure/Mask pressure
- Snore signal
- Audio and snoring channel
- 2 bipolar channels (PLM or EKG or EMG or EEG)
- Position, Activity
- Light
- SpO2, Pulse, Plethysmography
- Bipolar Channels: Touch-proof 1 mm keyhole connector, ±1024 mVp-p input range AC, <3 μVrms noise
- Flow/Pressure Signal: ± 100 cmH2O pressure input range, DC-80Hz, 200 Hz sampling frequency, <1 mmH2O noise
- Activity/Position Signals: Internal 3 axis, ±2 g
- Microphone: Internal 3.5 kHz bandwidth, 8000Hz sample rate, 16-bit ADC
- Wireless Interface: Bluetooth® V5.0 Low Energy wireless interface for external devices
- Storage Capacity: 4 GB
- Recording Time: 24 hours with 1xAA Battery (new Lithium battery)
- PC Communications: USB 2.0 hi-speed
- Power Source:
- One 1.5V AA battery during recording;
- Host PC USB during data download
- Battery Type: Alkaline primary, Lithium primary, nickel-metal hydride rechargeable (NiMH)
- Battery Cover: Tamper proof and locked
- Device Dimensions: 68 mm W x 62 mm H x 26 mm D
- Weight: 65 grams ± 5 without battery
- Display: Type OLED—Dimensions 19 x 35 mm, resolution 128 x 64 dots
- USB 2.0 Connection: USB-C
Minimum PC Requirements:
- Operating System: Windows® 8 and higher
- Processor: X64 based Intel or AMD, 1.7 GHz or faster
- Memory: 2 GB RAM, 4 GB of free disk space
- Resolution: 1024 x 768 or higher
Please refer to the user guides for relevant information related to any warnings and precautions to be considered before and during use of the products.
References:
1. Ragnarsdóttir, H., Már Þráinsson, H., Finnsson, E., Gunnlaugsson, E., Ægir Jónsson, S., Skírnir Ágústsson, J., & Helgadóttir, H. (2019). BodySleep: Estimating sleep states from respiration and body movements. [Poster Presentation]. World Sleep, Vancouver.
2. Dietz-Terjung, S., Martin, A., & Schoebel, C. (2020). A novel algorithm for the estimation of sleep states based on breathing and movement. Sleep (43, Issue Supplement 1), pp. A170-A170, DOI: https://doi.org/10.1093/sleep/zsaa056.442
3. Danker‐hopfe, H., Anderer, P., Zeitlhofer, J., Boeck, M., Dorn, H., Gruber, G., … & Saletu, B. (2009). Interrater reliability for sleep scoring according to the Rechtschaffen & Kales and the new AASM standard. Journal of sleep research, 18(1), 74-84.
4. Tataraidze, A., Anishchenko, L., Korostovtseva, L., Kooij, B. J., Bochkarev, M., & Sviryaev, Y. (2015, August). Sleep stage classification based on respiratory signal. In 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (pp. 358-361). IEEE.