COVID-19 FAQs
Looking for ResMed information on COVID-19? Discover our regularly updated FAQ page. If you need more answers, you can contact our customer service team and submit your questions.
FAQ - Responses to patient questions about coronavirus
ResMed manufactures a range of ventilators; They are indicated for home and hospital use, and have the flexibility for use in various clinical scenarios to support patients with respiratory insufficiency and failure. Our ventilators can provide invasive or non-invasive ventilation:
- Noninvasive ventilation (NIV) is a form of mechanical ventilation where air is delivered to the patient through a mask or mouthpiece.
- Invasive ventilation is used when sufficient ventilation cannot be achieved using non-invasive methods; air is delivered through a tube inserted into the trachea either by intubation or tracheotomy.
If you have been tested and diagnosed with coronavirus, you need to follow the recommendation of the medical staff that provided the diagnosis. At ResMed, we only sell our ventilation devices through the healthcare system of each country when there is a prescription from a physician. The therapy required to treat the worst cases of coronavirus is very complex and requires medical expertise and clinically trained staff to administer it. Your medical professional has the expertise to decide what kind of therapy is required for each patient.
If you have been tested and diagnosed with COVID-19, you need to follow the recommendation of the medical staff that provided the diagnosis. At ResMed, we only sell our ventilation devices through the healthcare system of each country. The therapy required to treat the worst cases of COVID-19 is very complex and requires medical expertise and clinically trained staff to administer it. Your medical professional has the expertise to decide what kind of therapy is required for each patient.
ResMed’s primary focus is to put as many lifesaving respiratory devices as possible into the hands of frontline heroes – the healthcare providers treating patients worldwide impacted by the COVID-19 pandemic.
Since the COVID-19 crisis began, the industry is facing unprecedented global demand for ventilators and other respiratory devices. We are working closely with hospitals, physicians, and government officials worldwide to understand their specific needs and have committed to tripling our global production of ventilators and bilevel devices to maximize the number of patients who get the help they need.
ResMed sincerely appreciates the interest and ideas offered by other companies, government agencies, health systems, clinicians, and academic and security researchers. There are reports that CPAPs might be modified to serve as ventilators. While technically it is possible to convert CPAPs to bilevel devices, it would take valuable time and resources to test and validate modifications to ensure we are delivering safe and effective devices that meet regulatory requirements during the COVID-19 pandemic. In addition, the hospitals, health systems, and governments who are working directly with ResMed to prioritize their needs are not requesting modified single-purpose CPAP devices that are commonly used in the home to treat sleep apnoea. For these reasons, we have prioritized the production of devices that address the immediate needs of hospitals and what clinicians are asking for.
Instead of modifying CPAP devices that are needed and used by sleep apnoea patients, we’ve modified our manufacturing process, shifting teams and tools to maximize the production and delivery of the devices healthcare providers are telling us they need most – ventilators and bilevel machines. We can already build them at scale without further research and testing, and without putting patients’ safety and lives at risk.
There is another very important way that those millions of home CPAP devices can be used to save lives. They should be used as prescribed by those who rely on them for the treatment of their sleep apnoea. If you have one, I urge you to use it. I’ve used mine faithfully, every night for many years.
Patients in need of more complex respiratory support need a wholly different device. ResMed has already ramped up production of such devices, and together with others in the industry, we will not stop until there is a ventilator or bilevel device for every patient worldwide who needs one.
Coronavirus doesn’t change your sleep apnoea diagnosis or treatment(s) (such as CPAP therapy), and you can continue to use your CPAP machine. If you have been diagnosed with coronavirus, you need to follow the medical recommendation you received from your physician.
A CPAP machine is intended to be a therapy for sleep apnoea syndrome. Some of the worst cases of coronavirus may lead to respiratory failure, and require other, specific ventilation devices which are intended to be used for respiratory failure.
ResMed is a manufacturer of medical devices. Please contact your medical professional for any advice concerning coronavirus and your CPAP treatment.
ResMed is a manufacturer of medical devices. Please contact your medical Professional for any advice concerning coronavirus and the use of oxygen with your CPAP treatment
Recommendations on disinfection processes for our therapy devices have been provided to your healthcare professionals.
A CPAP machine is intended to be a therapy for sleep apnoea syndrome and does not prevent you from contracting Covid-19. Please refer to your national healthcare authority or your medical professional for more information about Covid-19 and its contamination prevention.
A CPAP machine is intended to be a therapy for sleep apnoea syndrome. Your CPAP machine helps to prevent your sleep apnoea and you are not at higher risk of catching Covid-19 if you use a CPAP device. Please refer to your medical professional for more specific information on your concern.
Your CPAP device has been prescribed for personal use only. A CPAP machine is a therapy for sleep apnoea syndrome. Some of the worst cases of coronavirus may led to respiratory failure, and require other, specific ventilation devices which are intended to be used for respiratory failure.
If you are being treated for COVID-19, you should share with your doctor or clinical team your pre-existing conditions and what devices you are already using and follow their advice. As described by the World Health Organization, the key risk for spreading COVID-19 is person-to-person transmission in close contact. The most important advice in controlling the spread of COVID-19 is washing your hands, good hygiene, and social distancing. Maintaining the cleanliness of your ResMed device according to the user guide is always a good practice to reduce infection risk to anyone else from any contaminated surfaces.
Disinfection processes have been provided to your healthcare professional.
If you wish to clean your CPAP machine, the device’s user guide explains how to clean it on a weekly basis. For detailed instructions on how to clean your mask and tubing, please refer to the device’s user guide, and the following steps:
- Wash the air tubing in warm water using mild detergent and hot water. Do not wash in a dishwasher or washing machine.
- Rinse the air tubing thoroughly and allow to dry out of direct sunlight and/or heat.
- Wipe the exterior of the device with a dry cloth.
Please note: Cleaning any device or mask in the manner recommended in the product’s user guide does not guarantee decontamination or disinfection. For more information, contact your healthcare professional.
Recommendations on disinfection processes for our therapy devices have been provided to your healthcare professionals.
If you wish to clean your CPAP machine, the device User Guide explains how to clean it on a weekly basis. For detailed instructions on how to clean your mask, please refer to the User Guide, and the following steps:1. Wash the air tubing in warm water using mild detergent. Do not wash in a dishwasher or washing machine. 2. Rinse the air tubing thoroughly and allow to dry out of direct sunlight and/or heat. 3. Wipe the exterior of the device with a dry cloth.
Please note, cleaning any device or mask in the manner recommended in the User Guide does not guarantee decontamination or disinfection. For more information, contact your healthcare professional.
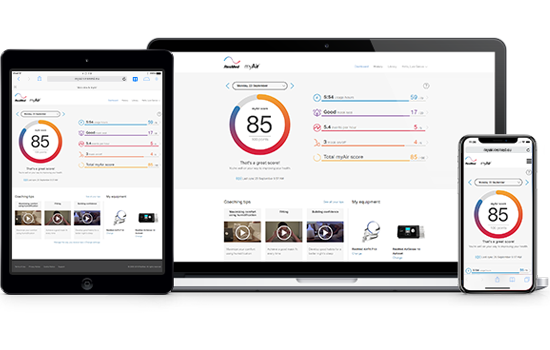
myAir app: a free CPAP therapy coaching app for sleep apnoea patients
During particularly busy times, physicians and healthcare providers aren’t always available for face-to-face chats to discuss CPAP therapy progress. To help you stay on track with your progress, download our free app, myAir. You’ll receive coaching tips and daily feedback, providing in-home support whenever you need it.
It’s easy to register:
- Create account. Click “Create account“ and fill in the required fields. If you already have a myAir account, you can sign in directly with your username and password.
- Activate your account. Check your inbox – you should receive an email from myAir asking you to activate your account. Click on the link in the email, then follow the prompts to create your password. If you can’t see an activation email in your inbox, please check your spam folder.
- 2-Step Verification. To access myAir, you have to enter your log-in information (step 1) and then enter a one-time passcode that is sent by email (step 2).
Note: To switch off the one-time passcode option and log in directly in the future, tick “Remember this device”. - Add your equipment.
Follow the prompts on your app screen and select your device AirSense 10/AirCurve 10 or AirSense 11:
If you have an AirSense 10 or an AirCurve 10
Enter the serial and device numbers for your Air10 machine – you’ll find them on the back of your device – and information about your mask. If you don’t have your CPAP equipment right now, you can complete your registration later.
If you have an AirSense 11
Enter the 4-digit key displayed on the AirSense 11 touchscreen to complete Bluetooth™ pairing. Bluetooth must be turned on in your smartphone settings.
Add mask
Select brand and model.
- Get started! Congratulations – you’re ready to start your myAir experience! Check your myAir score each morning to track your sleep therapy progress!
myAir is only available for the following ResMed devices:
If you need help with myAir, please visit the myAir Support page in your myAir app. It has lots of information about using myAir, including FAQs on account, password, data and more.
Join the ResMed Community
Stay connected and you’ll be among the first to hear all the latest news and developments.
Please refer to the user guide for relevant information related to any warnings and precautions to be considered before and during use of the product.